Spring 2023 (Volume 33, Number 1)
How to Manage
Retroperitoneal Fibrosis?
By Vandana Ahluwalia, MD, FRCPC; Luke Y. Chen, MD, FRCPC, MMEd;
and Mollie Carruthers, MD, FRCPC
Download PDF
|
Patient Case:
|
A 42-year-old man of Iranian descent presented originally with peri-umbilical discomfort. He had a computed tomography
(CT) scan in Iran showing soft tissue attenuation surrounding the aorta from the infrarenal vessels to the common
iliac bifurcation. This mass measured 5.7 cm x 3.0 cm x 5.9 cm. He received a course of prednisone empirically but,
when he was completely weaned off steroids, his mass recurred. He underwent a CT-guided biopsy which showed
chronic inflammation enriched with lymphocytes, plasma cells and eosinophils, consistent with retroperitoneal fibrosis.
He was restarted on prednisone and then placed on mycophenolate which he continued taking at presentation
to us.
His labs in Canada showed normal kidney function, with a creatinine of 81 μmol/L (normal 60-155 μmol/L) and an estimated
glomerular filtration rate of 103 ml/min (normal is >59 ml/min). His complete blood count, electrolytes, liver enzymes,
and urinalysis were normal. His C-reactive protein (CRP) was elevated at 19.1 mg/L (normal <3.1 mg/L). His total
protein was elevated at 84 g/L (normal 62-80 g/L). His IgG4 level was normal at 0.719 gram/L (normal 0.052-1.25 g/L).
The patient was referred for recommendations on the diagnosis and management of his retroperitoneal fibrosis. |
Introduction
Retroperitoneal fibrosis (RPF) was first described by John
Ormond, an American urologist in 1948.1 He described
it as sclerotic tissue in the retroperitoneum, commonly
peri-aortic or peri-iliac, and encasing adjacent structures.
Common presenting symptoms include abdominal, back
and/or flank pain along with constitutional symptoms.2
Patients may also present with acute renal failure due to
ureteric obstruction, with retroperitoneal fibrosis found
on abdominal/pelvis CT scanning. Peripheral edema may
also be present due to compression of the iliac veins in
the pelvis.3
RPF is a rare disease; for example, a Dutch study reported
an incidence of 1.3/100,000.2 There is a male
predominance, and the median age of onset is 64 years
old.2 Patients were historically managed by urology with
serial monitoring for hydronephrosis and serial ureteral
stenting.3
A definition of RPF by Scheel et al. has been proposed
that is not reliant on pathology. This includes:
1) identification by computed tomography (CT) or
magnetic resonance imaging (MRI) of a soft-tissue density
surrounding the infrarenal or iliac vessels; 2) absence
of aneurysmal dilation of the infrarenal aorta; 3)
absence of an intra-abdominal or pelvic mass; 4) lack
of suspicion of malignancy from history and physical
examination; and 5) negative age-appropriate cancer
screening.4 A radiographic-focused definition is important
but does not precisely address the etiology of RPF,
as management differs depending on the underlying
cause. The pattern of aortic involvement, ureteric involvement,
presence of lymph nodes or extension into
the pelvic wall are not predictive radiographically of
the underlying disease.5
What Are the Causes of RPF?
The pathogenesis of RPF is not known.6 However, when
pathology is obtained from surgical or CT-guided biopsies,
certain patterns emerge. Unfortunately, there have
been no prospective analysis of biopsy results in retroperitoneal
fibrosis. There is a high prevalence of both idiopathic
and IgG4-related RPF in cases of RPF reported.5
In Khosroshahi’s study, any patients who carried a prior
diagnosis of malignancy were excluded. This fits with the
literature in which the association or causal relationship
of RPF and malignancy is hard to quantify.6 It is safe to
say that IgG4-related disease is the cause of RPF 30-57%
of the time.6 Determining whether the patient has IgG4-
related disease may represent one of the most important
considerations, as it is a systemic disease that may progress
over time.
There have been associations proposed between RPF
and other conditions such as atherosclerosis, certain medications,
and connective tissue disease.3 In cases where
the tissue clearly shows one of these conditions, then one
can make a case for causality. This occurs with lymphoma,
for example, where the retroperitoneal soft tissue infiltration
shows B-lymphocyte clonal proliferation.7 Similarly,
when the adventitia of the aorta shows granulomatous
infiltration and antineutrophil cytoplasmic antibodies
are positive, this is consistent with granulomatosis with
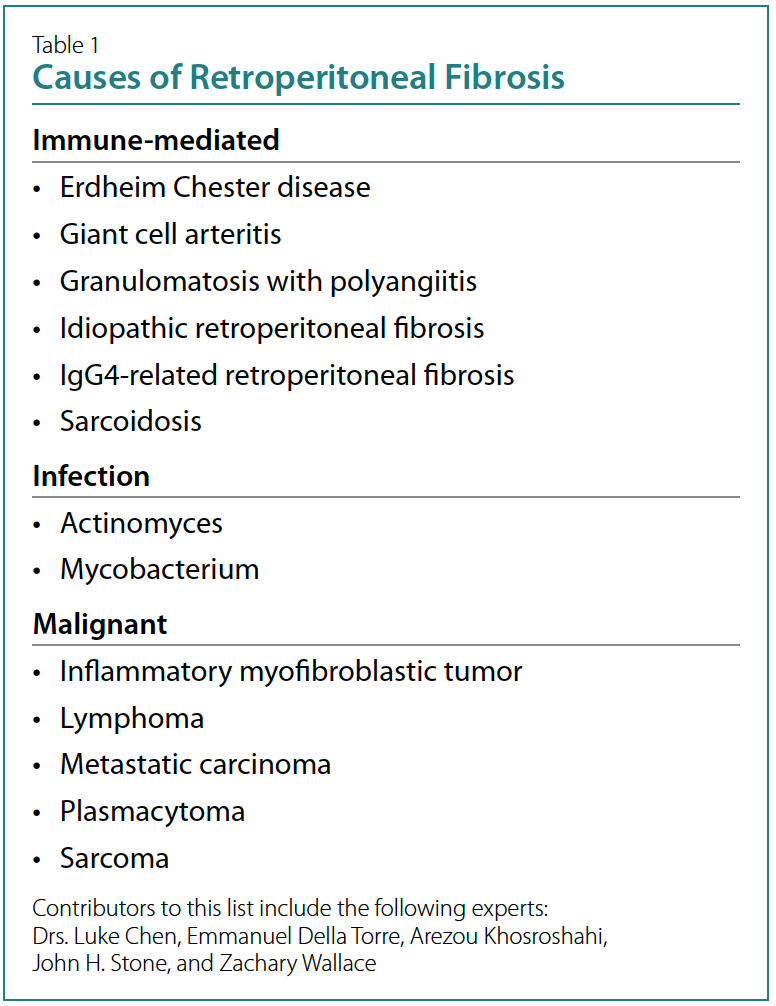
polyangiitis.8 The list in Table 1 represents a more tailored
differential diagnosis for RPF based on histopathologic
evidence and expert opinion.
What Is the Appropriate Work-up of RPF?
Due to the high prevalence of IgG4-related disease with
retroperitoneal fibrosis, a full clinical work up for systemic
IgG4-related disease makes sense (Table 2). The
systemic nature of IgG4-related disease makes pattern
recognition difficult when making a clinical diagnosis.
Similarly, the IgG subclasses are an unreliable biomarker
for diagnosis, with a specificity of 90% and sensitivity
of 60% in one single centre study.9 The typical
pattern of IgG4-related disease has been defined
in recent American College of Rheumatology (ACR)/
European Alliance of Associations for Rheumatology
(EULAR) classification criteria.10 However, these classification
criteria remain heavily reliant on histopathology
and, therefore, most cases of IgG4-related disease
require biopsy confirmation.11 IgG4-related RPF is defined
by storiform fibrosis and enrichment of IgG4-positive
plasma cells with a IgG4/IgG ratio of >40%. By
contrast, idiopathic RPF shows lymphoid follicles,
extensive non-storiform fibrosis and a low IgG4/IgG
ratio. The idiopathic RPF patients also have no extraabdominal
involvement.
In RPF, as in IgG4-related disease, a CT-guided or surgical
biopsy is often necessary. If there is another organ
site that is suggestive of IgG4-related disease, such as a
submandibular gland, then the more easily accessed organ
would be biopsied preferentially. Given the various
causes of RPF listed in Table 1, management would be
very different, depending on whether an immune-mediated
condition, malignancy or infection is present.
The index patient was assessed in clinic for IgG4-
related disease. He was asymptomatic at that time. His
physical exam was notable for bilateral submandibular
swelling. On CT scan of his neck, chest, abdomen, and
pelvis he was found to have bilateral submandibular
gland enlargement, and bilateral mediastinal and hilar
lymphadenopathy. The RPF mass had decreased in size
and now measured 5.1 cm x 2.7 cm x 5.2 cm. Figure 1
shows the characteristic axial view of RPF showing soft
tissue encasement of the abdominal aorta at the level of
the renal vessels. Histopathology slides of the retroperitoneal
mass that he brought from Iran were reviewed at
the British Columbia Cancer Agency. The pathology was
found to be diagnostic of IgG4-related disease, notably
with lymphoplasmacytic infiltration, storiform fibrosis
and obliterative phlebitis with an IgG4/IgG ratio of
>40%. This patient was diagnosed as having IgG4-related
disease after this biopsy review.
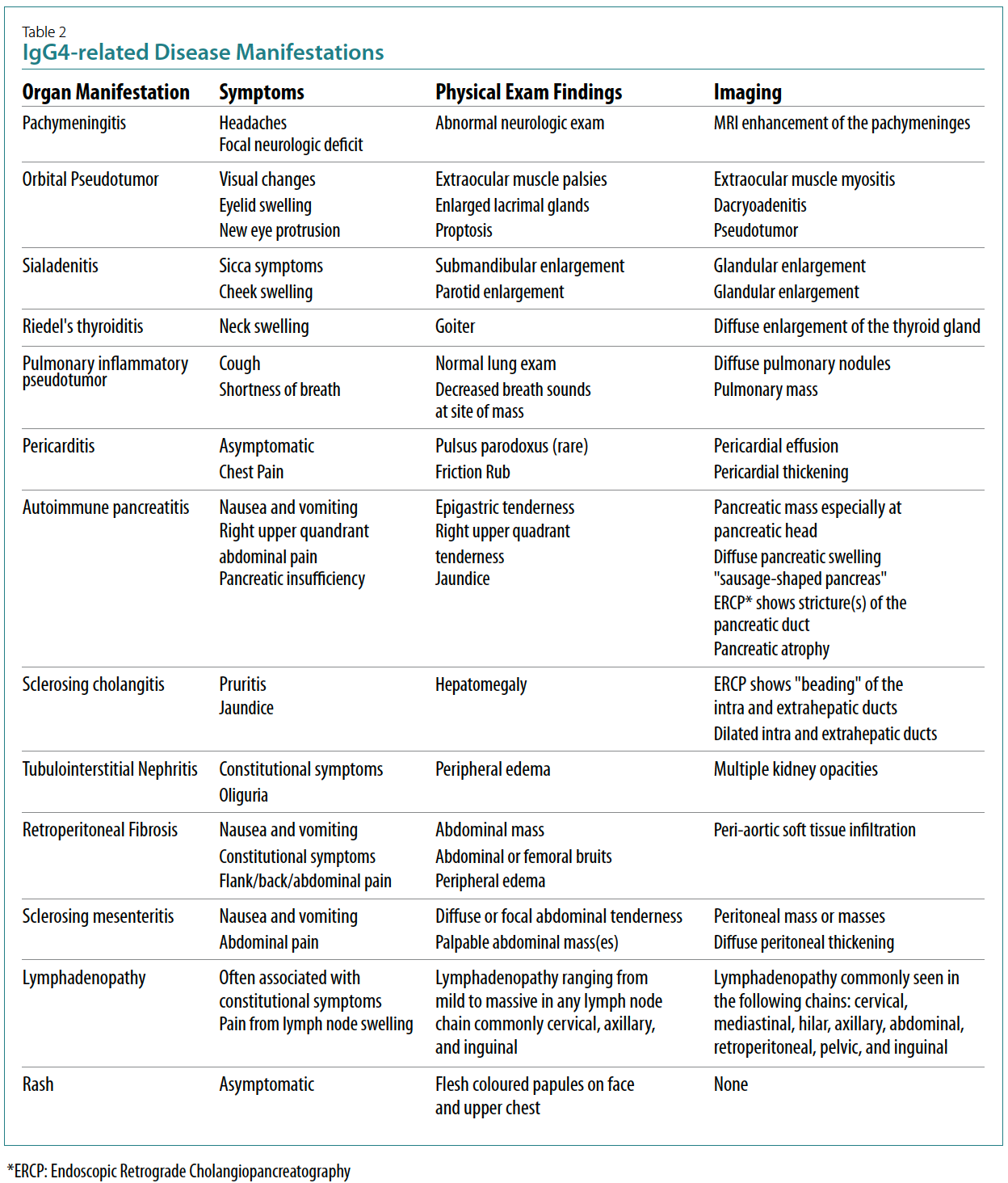
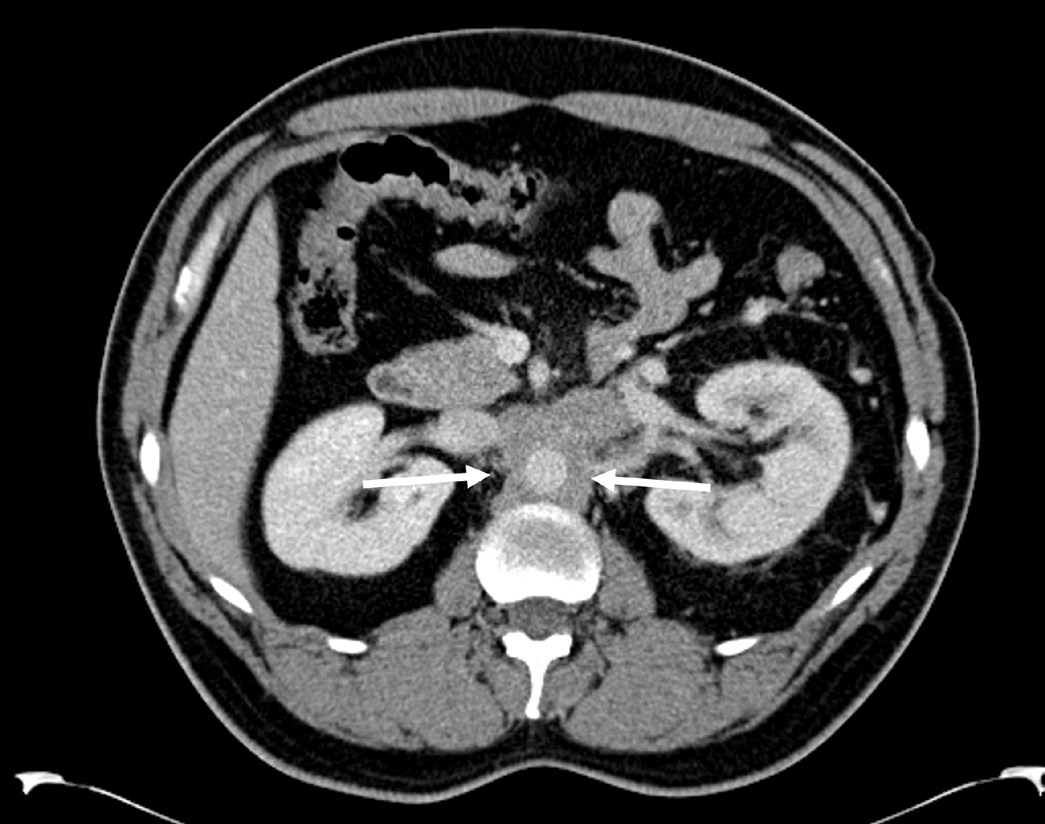
Figure 1: Axial CT with contrast showing maximal 2.5 cm
soft tissue thickening encasing the aorta indicated
between 2 white arrows.
What is the Appropriate Management of
IgG4-related RPF?
The proper treatment of IgG4-related disease is emerging.
The mainstay of therapy is prednisone.12 Other options
for IgG4-related disease include disease modifying anti-rheumatic drugs, of which mycophenolate is the most
favoured.13,14 Rituximab has been observed to be effective
in both IgG4-related disease and idiopathic retroperitoneal
fibrosis.15,16,17 Ongoing studies are being done
with respect to newer agents specifically for IgG4-related
disease.
The index patient was refractory to both prednisone
and mycophenolate. He was commenced on rituximab,
but after one course of treatment, he was lost to follow up.
Vandana Ahluwalia, MD, FRCPC
Rheumatologist,
William Osler Health System
Brampton, Ontario
Luke Chen, MD, FRCPC, MMEd
Clinical Associate Professor of Hematology,
University of British Columbia
Vancouver, British Columbia
Mollie Carruthers, MD, FRCPC
Rheumatologist and Clinical Investigator,
Arthritis Research Canada
Vancouver, British Columbia
References:
1. Ormond JK. Bilateral ureteral obstruction due to envelopment and compression by an inflammatory
retroperitoneal process. J Urol. 1948; 59(6):1072-9.
2. Van Bommel EF, Jansen I, Hendriksz TR, et al. Idiopathic retroperitoneal fibrosis: prospective
evaluation of incidence and clinicoradiologic presentation. Medicine. 2009; 88(4):193-201.
3. Vaglio A, Salvarani C, Buzio C. Retroperitoneal fibrosis. Lancet. 2006; 367(9506):241-51.
4. Scheel PJ Jr, Feeley N. Retroperitoneal fibrosis: the clinical, laboratory, and radiographic presentation.
Medicine. 2009; 88(4):202-7.
5. Khosroshahi A, Carruthers MN, Stone JH, et al. Rethinking Ormond's disease: "idiopathic" retroperitoneal
fibrosis in the era of IgG4-related disease. Medicine (Baltimore). 2013; 92(2):82-91.
6. Rossi GM, Rocco R, Accorsi Buttini E, et al. Idiopathic retroperitoneal fibrosis and its overlap with
IgG4-related disease. Intern Emerg Med. 2017; 12(3):287-99.
7. Wan N, Jiao Y. Non-Hodgkin lymphoma mimics retroperitoneal fibrosis. BMJ Case Rep. 2013.
8. Izzedine H, Servais A, Launay-Vacher V, et al. Retroperitoneal fibrosis due to Wegener's granulomatosis:
a misdiagnosis as tuberculosis. Am J Med. 2020; 113(2):164-6.
9. Carruthers MN, Khosroshahi A, Augustin T, et al. The diagnostic utility of serum IgG4 concentrations
in IgG4-related disease. Ann Rheum Dis. 2015; 74(1):14-8.
10. Wallace ZS, Naden RP, Chari S, et al; Members of the ACR/EULAR IgG4-RD Classification Criteria
Working Group. The 2019 American College of Rheumatology/European League Against Rheumatism
classification criteria for IgG4-related disease. Ann Rheum Dis. 2020; 79(1):77-87.
11. Chen LYC, Mattman A, Seidman MA, et al. IgG4-related disease: what a hematologist needs to
know. Haematologica. 2019; 104(3):444-455.
12. Masaki Y, Matsui S, Saeki T, et al. A multicenter phase II prospective clinical trial of glucocorticoid
for patients with untreated IgG4-related disease. Mod Rheumatol. 2017; 27(5):849-854.
13. Khosroshahi A, Wallace ZS, Crowe JL, et al. Second International Symposium on IgG4-Related
Disease. International Consensus Guidance Statement on the Management and Treatment of
IgG4-Related Disease. Arthritis Rheumatol. 2015; 67(7):1688-99.
14. Yunyun F, Yu P, Panpan Z, et al. Efficacy and safety of low dose mycophenolate mofetil treatment
for immunoglobulin G4-related disease: a randomized clinical trial. Rheumatology (Oxford). 2019;
58(1):52-60.
15. Carruthers MN, Topazian MD, Khosroshahi A, et al. Rituximab for IgG4-related disease: a prospective,
open-label trial. Ann Rheum Dis. 2015; 74(6):1171-7.
16. Boyeva V, Alabsi H, Seidman MA, et al. Use of rituximab in idiopathic retroperitoneal fibrosis. BMC
Rheumatol. 2020; 4:40.
17. Wallwork R, Wallace Z, Perugino C, et al. Rituximab for idiopathic and IgG4-related retroperitoneal
fibrosis. Medicine (Baltimore). 2018; 97(42):e12631.
|
