Fall 2023 (Volume 33, Number 3)
Survey Results:
Medication Access Issues
in Canadian Rheumatology
Download PDF
This edition’s Joint Count survey, in collaboration
with the CRA Stakeholder Engagement Committee,
asked CRA members about medication access
issues in Canadian rheumatology. While medication access
issues and shortages have been a concern in the past,
these issues have become more prevalent and widespread
since the pandemic. As one survey respondent pointed
out “Access to biologic treatment is a daily issue in pediatric
rheumatology, with problems related to limited
provincial coverage for medication (refusal to cover medications
for which a randomized controlled trial [RCT] is
not available), resulting in needing to request 'special access'
with a long letter for each request and renewal, and
the secondary requirement to complete pages of health
insurance forms, often not set up for pediatric needs, requiring
handwritten explanations.”
Indeed, approximately eighty percent of respondents
reported that they had encountered medication shortages
and access issues, though the frequency of these issues varied
from 1-3 times a year for some to almost every month
for others.
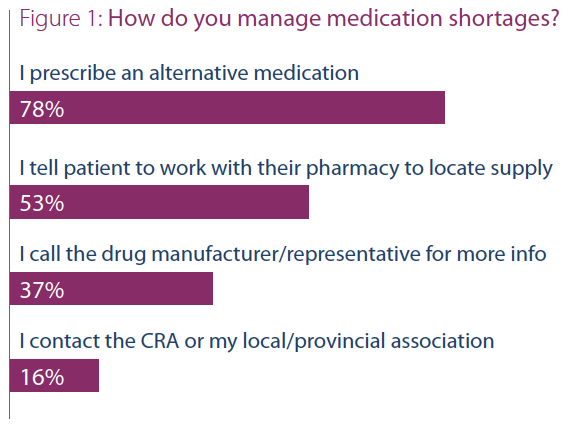
When queried as to how they managed medication
shortages, the most common response selected was “I
prescribe an alternative medication” (~78%); this was
followed by “I tell patients to work with their pharmacy
to locate supply” (~53%), followed by “I call the drug
manufacturer/representative for more info” (~37%), and
finally, “I contact the CRA or my local/provincial association”
(~16%). One respondent also highlighted turning to
patient support programs.
The final question asked readers “What was the most
recent medication shortage or medication access issue
of importance for you?” The specific medications and
concerns mentioned include the following:
- Rituximab (one of the biggest issues for access in
situations where it is the optimal medication for rare
diseases, though even when compassionate access is
granted — which it often is, thankfully — the process
to go through can lead to delays (e.g. waiting for
insurance to decline it, etc.)
- Triamcinolone hexacetonide (for joint injection in
children)
- Prazosin for Raynaud's phenomenon
- Depo-Medrol®
- Oral suspension naproxen
- Methotrexate prefilled (subcutaneous)
- Tacrolimus
- Folate 5 mg
- Pediatric doses of etanercept (25 mg)
- Adalimumab biosimilars (some brands)
- Tocilizumab
- Prednisone (5 mg)
- Quinacrine (an alternative antimalarial)
- Mycophenolate mofetil coverage (for interstitial lung
diseases [ILD] in systemic sclerosis)
- Difluprednate for uveitis
- Upadacitinib
- Chloroquine (a big issue for lupus patients
who do not tolerate or have side effects from
hydroxychloroquine)
- Sulfasalazine (shortages for enteric-coated
formulation and non-enteric-coated)
- Anakinra
- Leflunomide (20 mg)
- Avacopan and abatacept (for CTLA-4
haploinsufficiency)
- Biologic access in general is difficult for patients with
no provincial health care program eligibility
Note that a total of 53 completed surveys were received
out of a possible 617. For further information on this topic
or for any questions, please reach out to Sarah Webster
at swebster@rheum.ca. The CRA Stakeholder Engagement
Committee also welcomes your feedback.
|




