Winter 2016 (Volume 26, Number 4)
CRA 2016 Access to Medications Survey
Download PDF
Access to medications is a serious concern for many patients and their healthcare professionals. Challenges in accessing medications are not only frustrating, but can even be a matter of life and death.
For this issue’s Joint Count survey, we asked CRA members to rate their major and minor frustrations related to accessing medications for their patients, and to tell us which specific medications were difficult to access.
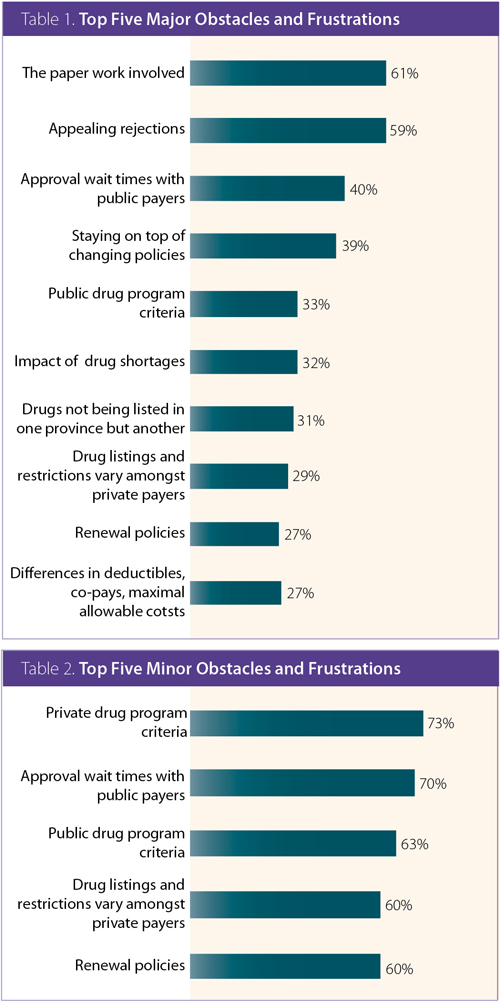
According to the 129 respondents, the top four major obstacles and/or frustrations included the paper work involved (61%), appealing rejections (59%), approval wait times with public payers (40%), followed closely by staying on top of changing policies (39%); refer to Tables 1 and 2 for further details.
Other concerns related to drug access cited by respondents included: drugs for rare diseases; off-label uses; the lag time between approval of drugs in other countries and Canada; and the difficulties of access to medications for pediatric patients.
When asked to list specific medications that are challenging to access for their patients, an overwhelming majority of CRA members mentioned rituximab, apparently due to the number of conditions for which there is a lack of approved indication and/or randomized controlled trials (RCTs). With regard to rituximab for granulomatosis with polyangiitis (GPA), one respondent explained “Patients with severe disease cannot wait the length of time public funding approval takes. Even more frustrating is the unwillingness to cover rituximab for maintenance, despite good evidence it is superior to currently available therapies.” Rituximab was also reported as difficult to access for a number of other conditions, including rheumatoid arthritis and lupus.
Many also stated that biologics as a class were difficult to access; specific biologics that were mentioned include tocilizumab, adalimumab, belimumab, canakinumab, and anakinra. Others reiterated that most drugs for orphan diseases and off-label uses were also challenging to access.
Indeed, difficulties in accessing medications are a major issue in healthcare. The CRA and Ontario Rheumatology Association established a first when they negotiated an understanding with private payers, through the Canadian Life and Health Insurance Association (CLHIA), of a common criteria for use of biologic agents in RA. The CRA Therapeutics Committee is keen to receive documentation of challenges that present themselves, in order to present real examples to the appropriate agencies.
Indeed, it will take collaboration on all sides to improve access and, ultimately, the quality of life for patients.
|